Coronavirus Update- ICMR Gives Guidelines For 17 New Kits To Detect COVID-19
By: Priyanka Maheshwari Sun, 29 Mar 2020 1:39:42

On Saturday, Indian Council of Medical Research (ICMR) issued guidelines for use of commercial kits for nasal and throat swab based diagnosis of COVID-19 in India.
According to ICMR, Currently, RT-PCR probes for diagnosis of COVID-19 are procured from USA by ICMR-NIV and are distributed to the testing laboratories across the country. ICMR also said that it welcomes use of commercial kits for diagnosis of COVID-19.
In a statement ICMR said, "US FDA EUA/CE IVD approved kits can be used directly after due approval from DCGI and intimation to ICMR." The ICMR has also established a fast-track mechanism for validation of non US FDA EUA/CE IVD approved kits at ICMR NIV. Test kits with 100% concordance among true positive and true negative samples will be approved for commercial use in India.
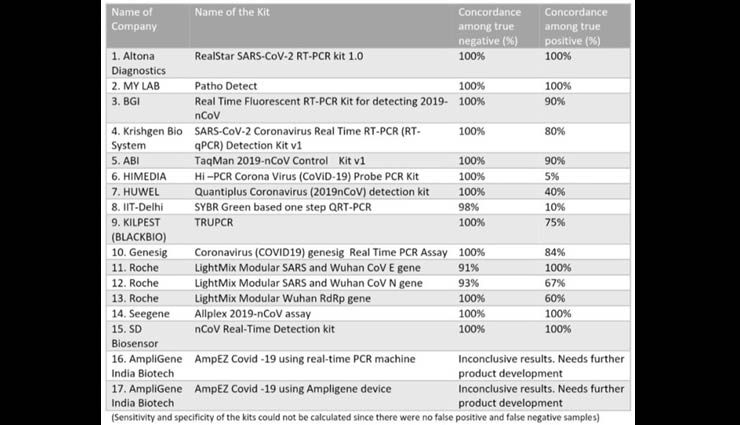
ICMR also said that it has completed evaluation of 17 kits. The ICMR also released the results of the validation of these 17 kits.
The number of coronavirus cases across the world rose to 640,589 as on Saturday evening, with the US leading with 115,547 cases, while the global death toll rose to 30,249 according to data from the Johns Hopkins University's Coronavirus Resource Centre.
Italy, with 10,023 fatalities, comprised over one third of the death toll, and was followed by Spain with 5,812 and China's Hubei with 3,177. Iran with 2,517 deaths, and France with 2,314 were joined in the four-figure category by the UK, where the toll is now 1,019. As regards the total number of cases, the US was followed by Italy (92,472), China (81,999), Spain (72,248), Germany (56,202), Iran (35,408), France (33,450) and the UK (17,301).





